Pharmaceutical Sector Update – November 2020
CURRENT DEVELOPMENTS – GLOBAL
J&J Covid-19 Vaccine candidate yields strong immune response in early trial
A single dose of Johnson & Johnson’s experimental COVID-19 vaccine produced a strong immune response against the novel coronavirus in an early-to-mid stage clinical trial. The vaccine, called Ad26.COV2.S was equally well-tolerated at two different doses.
However, it is unclear whether elderly people, one of the populations most at risk from the virus, will be protected to the same degree as younger people with the J&J vaccine. The trial in close to 1,000 healthy adults, which is backed by the US government, began after the J&J vaccine was found in July 2020 to offer strong protection in a single dose to monkeys.
Phase III trials of Astrazeneca Covid-19 Vaccine candidate begins in US
AstraZeneca said it has begun enrolling adults for a US-funded, 30,000-subject late-stage study of its high profile COVID-19 vaccine candidate. Trial participants will receive either two doses of the experimental vaccine, dubbed AZD1222, four weeks apart, or a placebo.
The trial is being conducted under the US government’s Operation Warp Speed program, which aims to accelerate development, manufacturing and distribution of vaccines and treatments for COVID-19.
Bacterial viperins prove effective against human viruses: researchers
Virus-fighting viperins, part of the human immune system, turn out to have bacterial counterparts that might boost the fight against human disease. By tracking the evolution of what may be our oldest means of fighting off viral infection, a group at the Weizmann Institute of Science has uncovered antiviral substances that may lead to the development of highly effective antiviral drugs.
These substances are made by virus-fighting enzymes known as viperins, which were previously known to exist only in mammals, and have now been found in bacteria. The molecules produced by the bacterial viperins are currently undergoing testing against human viruses such as the influenza virus and COVID-19. The study was published in Nature.
Gilead gets approval for Jyseleca (Filgotinib) in Japan for Rheumatoid Arthritis
Gilead Sciences announced that the Japanese Ministry of Health, Labour and Welfare has granted Gilead KK (Tokyo, Japan) regulatory approval of Jyseleca (filgotinib 200 mg and 100 mg tablets), a once-daily, oral, JAK1 preferential inhibitor for the treatment of rheumatoid arthritis (RA) in patients who have had an inadequate response to conventional therapies, including the prevention of structural joint damage.
Gilead Japan will hold the marketing authorisation of Jyseleca in Japan and will be responsible for product supply of Jyseleca in Japan, while Eisai will be responsible for product distribution of Jyseleca in Japan in RA. The companies will jointly commercialise the medicine to make it available to physicians and patients across Japan.
Curevac links Covid-19 Vaccine to immune response, setting it up to enter pivotal trial
CureVac has shared phase 1/2 data on its COVID-19 vaccine candidate CVnCoV. The German biotech said the mRNA vaccine dose selected for further development sparked increases in virus-neutralizing antibodies and early indications of T-cell activation. While BioNTech and Moderna raced mRNA vaccines into the clinic, CureVac advanced its candidate into human testing more slowly, albeit still at a breakneck speed by historical standards.
CureVac used the extra time in preclinical to design a differentiated asset. Rather than build upon chemical modification of the RNA, CureVac optimized the nucleic acid itself. The result is an mRNA vaccine that CureVac expects to generate a balanced immune response and be more straightforward to scale up. The phase 1 data provide an early, incomplete look at whether the candidate can live up to those expectations.
Global opioid use disorder market to reach $3.7 bn by 2028
The opioid use disorder (OUD) market is expected to grow from $1.6 billion in 2018 to $3.7 billion by 2028 across the eight major markets (8MM) at a compound annual growth rate (CAGR) of 8.8 per cent, according to GlobalData.
The company’s latest report, ‘Opioid Use Disorder-Opportunity Analysis and Forecasts to 2028’, reveals that the main driver of growth will be the launch of reformulations of buprenorphine, in particular extended-release formulations including Indivior’s Sublocade and Camurus’ Buvidal.
Vivet, Pfizer sign pact for gene therapy to treat Wilson disease
Vivet Therapeutics and Pfizer announced that they have entered into a manufacturing agreement, under which Pfizer will provide clinical supply for a Phase 1/2 clinical trial evaluating Vivet’s proprietary, investigational gene therapy, VTX-801, for the potential treatment of Wilson disease, a rare and potentially life-threatening liver disorder. The trial is expected to commence in early 2021. Terms of the agreement were not disclosed.
CURRENT DEVELOPMENTS – INDIA
Pharma sector needs global collaboration
The need of global collaboration in the pharma sector from research and development to drug manufacturing has never been as great as it is now amid the deadly COVID pandemic. Ahead of the 14th edition of the annual BioPharma and Healthcare Summit, that bring stakeholders from India and the United States on one platform, officials and industry leaders said the global health crisis requires a global solution, which can be achieved through global collaboration. It was highlighted that India and the United States can play a lead role in this.
ICRA revises domestic pharma industry outlook to negative
Rating agency ICRA has revised its outlook on Indian pharmaceutical industry to negative from stable due to ongoing lockouts in parts of China following the outbreak of coronavirus. The domestic drug industry is highly dependent on imports, with more than 60 % of its API requirement being shipped from overseas locations, especially China.
In some specific active pharmaceutical ingredients (APIs), like cephalosporins, azithromycin and penicillin, the dependence is as high as 80-90 % of the total imports of APIs and intermediates into India, China accounts for 65-70%.
Indore SEZ exports log marginal decline in Apr-June amid Covid-19 pandemic
Exports from Indore Special Economic Zone (SEZ) have not been impacted much due to the coronavirus outbreak, with outward shipments dropping merely 1.14 per cent in April-June. The multi-product Indore SEZ logged exports worth Rs 2,555.42 crore in the first quarter of the ongoing fiscal as against Rs 2,584.88 crore in the corresponding period a year ago.
In this, 70 per cent contribution was of medicines, according to a union commerce ministry official. Despite the lockdowns imposed due to COVID-19, the production in pharma units continued as the government kept it under essential services category.
UCPMP should be made mandatory without further delay: experts
Recently, D V Sadananda Gowda, Minister of Chemicals and Fertilizers, informed that the Uniform Code for Pharmaceuticals Marketing Practices (UCPMP) is voluntary in nature and the Government has not yet decided to make it mandatory. This announcement did not go down well with patient activists and the medical fraternity.
Extending benefits to doctors through associations is unethical. But this is being flouted with impunity. The Medical Council of India (MCI) had amended the Indian Medical Council (Professional conduct, etiquette and Ethics) regulations, 2002 at its meeting on February 18, 2014, and exempted the "Professional Associations of Doctors” from the purview of Medical Ethics. Industry experts feel there is an urgent need to take steps to reverse this amendment of the MCI and make the UCPMP mandatory.
Govt. regulation may hamper pharma sector growth: Pfizer
Pharma major Pfizer fears that the pharma industry is expected to face challenges due to government regulations. "While implementation of large scale healthcare initiatives expected to provide a boost to the pharma industry, there will be continued challenges in the areas of price controls, ad-hoc regulatory changes and new policies that may impact predictability and sustainable growth," it further said.
PRODUCT APPROVALS / LAUNCHES - INDIAN COMPANIES
DRL launches Dimethyl Fumarate DR capsules in US
Dr Reddy’s has launched dimethyl fumarate delayed-release capsules, a therapeutic equivalent generic version of Tecfidera (dimethyl fumarate) delayed-release capsules for Multiple Sclerosis (MS), approved by the USFDA. The Tecfidera brand and generic market had US sales of approximately $3.8 billion MAT for the most recent twelve months ending in June 2020 according to IQVIA Health.
Lupin launches Divalproex Sodium ER Tablets
Lupin announced the launch of divalproex sodium extended-release (ER) tablets USP, 250 mg and 500 mg, having received an approval from the USFDA earlier. Divalproex sodium ER tablets USP is the generic equivalent of depakote extended release (ER) tablets of AbbVie indicated for Acute treatment of manic or mixed episodes associated with bipolar disorder (with or without psychotic features), Monotherapy and adjunctive therapy of complex partial seizures and simple and complex absence seizures; adjunctive therapy in patients with multiple seizure types that include absence seizures and Prophylaxis of migraine headaches.
Glenmark launches Nindanib (Nintedanib) for Pulmonary Fibrosis in India
Glenmark Pharma launched NINDANIB (Nintedanib 100 and 150 mg capsules) for the treatment of pulmonary fibrosis in India. Nintedanib is approved by the Indian drug regulator for the treatment of idiopathic (unknown cause) Pulmonary Fibrosis. In a recently published INBUILD trial, Nintedanib showed significantly lower annual rate of decline in FVC (Forced Vital Capacity) – a measure of lung health – with various progressive fibrosing interstitial lung diseases. Moreover, two clinical trials are being rolled out for to study the efficacy and safety of Nintedanib as a treatment of SARS-COV2 induced Pulmonary Fibrosis in moderate to severe COVID-19 patients,” informed the company through a statement.
Sun Pharma arm launches Plaque Psoriasis treatment drug in Japan
Sun Pharma's Japanese subsidiary has launched its specialty product Ilumya, indicated for treatment of Plaque Psoriasis in adult patients in Japan. It has got approval from the Japanese government. It is a subcutaneous injection (100 mg Syringe) for the treatment of Plaque Psoriasis in adult patients who have an inadequate response to conventional therapies. It is Sun Pharma's first innovative drug to be launched in the Japanese market.
Zydus Cadila gets nod for Fingolimod caps & Verapamil Hydrochloride injection
Zydus Cadila has received final approval from the USFDA to market Fingolimod Capsules, 0.5 mg (US RLD: Gilenya Capsules). Fingolimod is an immunomodulating drug. It is a sphingosine 1-phosphate receptor modulator indicated for the treatment of relapsing forms of Multiple Sclerosis (MS). The Company has also received the final approval from the USFDA to market Verapamil Hydrochloride Injection USP, 5 mg/2 mL (2.5 mg/mL) and 10 mg/4 mL (2.5 mg/mL), Single-Dose Vials (US RLD: Isoptin Injection). Verapamil injection is used to rapidly or temporarily restore normal heartbeats in people with certain heart rhythm disorders.
Indoco launches Favipiravir 400 mg tablets
Indoco Remedies announced the launch of FEVINDO (Favipiravir) 400 mg tablets in India. Fevindo – 400 is an antiviral drug, effective against the RNA-based influenza virus. The drug has been approved by DCGI in the treatment of COVID-19.
FINANCIAL PERFORMANCE OF SOME KEY PLAYERS
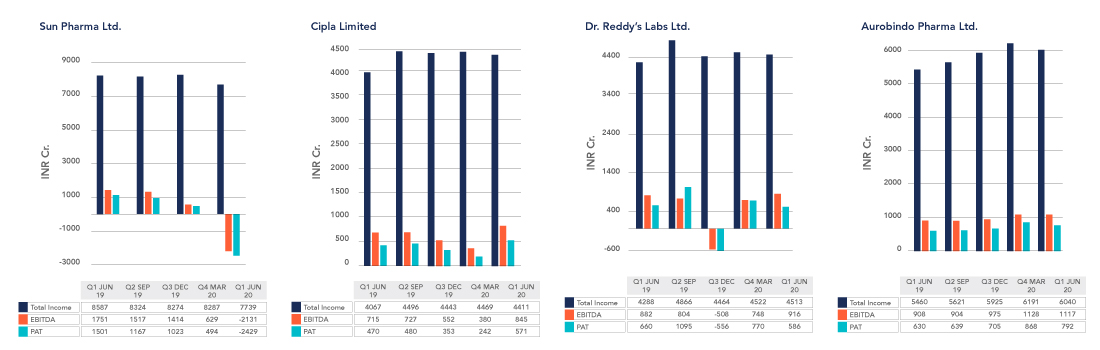
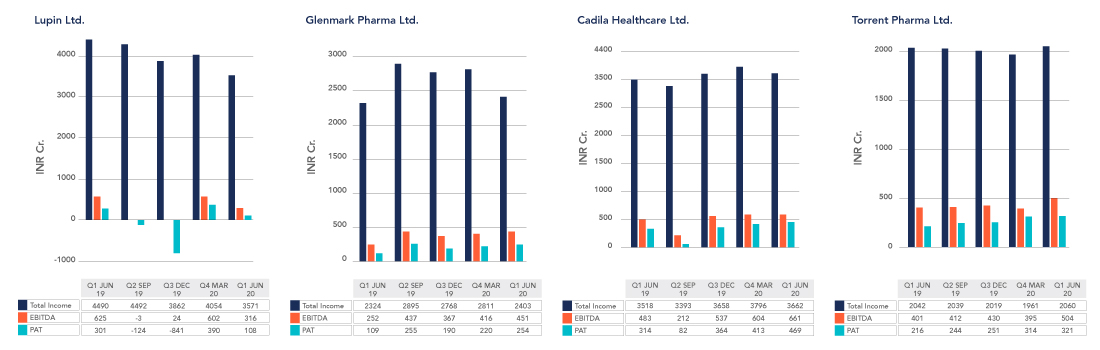
(Source: ET Markets, Moneycontrol, Company Financial Reports)
PEOPLE MOVEMENT
Nikhil Chopra is the new CEO of JB Chemicals and Pharmaceuticals
The Board of directors of JB Chemicals & Pharmaceuticals appointed Nikhil Chopra as Whole-time Director and Chief Executive Officer of the Company for a period of five years. Earlier, Nikhil Chopra worked as Head-India Business at Cipla. He holds M.Sc. (Organic Chemistry) from Gujarat University. For over two decades, he has spearheaded breakthrough ideas focused on creating greater access to high quality treatment and medicines, and gain a significant competitive advantage over peers, especially in therapies such as respiratory, urology HIV and paediatric care.
Biocon appoints Anupam Jindal as its CFO
Anupam will head the finance function at Biocon and be part of the Executive Leadership Team. He will report into the company’s CEO and MD, Siddharth Mittal. Prior to joining Biocon, Jindal worked with the Vedanta Group of companies for 22 years, where he held the position of Group CFO at Sterlite Technologies since 2006. He holds a Bachelor of Commerce (B Com) degree from ML Sukhadia University, Udaipur. He is also a Chartered Accountant from the Institute of Chartered Accountants of India and a Company Secretary.
Vaibhav Karandikar is the new CFO of Sanofi India
Sanofi India announced the appointment of Vaibhav Karandikar as Chief Financial Officer (CFO), effective October 6, 2020. Karandikar joined Sanofi 13 years ago. He is a Chartered Accountant, Cost Accountant & Company Secretary. He has held growing responsibilities in business controlling, accounting and in various M&A and transformation projects, for both commercial operations and manufacturing facilities. Prior to joining Sanofi, Vaibhav has worked with Sandoz India, Tata Power and Hindustan Ciba Geigy.
S Aparna appointed as Secretary, Department of Pharmaceuticals
S Aparna, an IAS officer from the Gujarat cadre 1988, has been appointed as Secretary, Department of Pharmaceuticals (DoP), under the Ministry of Chemicals and Fertilizers. She will succeed PD Vaghela, who demitted the office on his superannuation on September 30, 2020, as per an order issued by the Department of Personnel and Training. In 2017, she was appointed to the post of Executive Director, World Bank, representing the Constituency of India, Bangladesh and Sri Lanka. In 2019, she was given a proforma promotion as Additional Chief Secretary (ACS) while she was on deputation in Washington DC as Executive Director, World Bank.
Manjiri Gharat elected as VP of FIP for 2020 – 2024
Manjiri Gharat, VP & Chairperson, Community Pharmacy Division of Indian Pharmaceutical Association (IPA), has been elected as Vice President of the International Pharmaceutical Federation (FIP) for 2020 – 2024. She is the first woman pharmacy professional and second pharmacy professional from India to be elected as FIP Vice President. In the past, Praful Sheth had been VicePresident of FIP for the term 2006 – 2010 & 2010 2014. The International Pharmaceutical Federation (FIP) is a global body representing over four million pharmacists and pharma scientists.
Zydus wellness appoints Sandeep Anand as its new Chief Marketing Officer
Sandeep Anand was earlier the CMO for the Food Delivery business of Zomato. He focused on Offline marketing (Consumer Advertising), Digital (Performance Marketing, CRM, Growth Analytics, and Alliances. He is an alumnus of Jamia Milia Islamia & Management Development Institute (MDI). Sandeep joined Zomato in December 2018 from GSK Healthcare known for Horlicks. Sandeep has worked with GSK, Ranbaxy, and Reckitt Benckiser in his career.
Alind Sharma joins Optum as Vice President – Human Capital
Optum, a health services and innovation company, has announced the appointment of Alind Sharma as VP – Human Capital. In his earlier role, Alind was with Pfizer as Senior Director in charge of HR responsibilities across all Pfizer manufacturing sites in the APAC region. He has worked with Monsanto, Tata International, Glenmark Pharma and Ranbaxy. He is a Mechanical Engineer and completed his management studies at IIM Ahmedabad.
Indegene hires C-suite Executives from GE & Quikr
Healthcare technology company Indegene has brought on board Marut Setia, former chief marketing officer for GE’s South Asia region, as Senior VP-Emerging Markets and Devices. Atul Tewari, former COO of Quikr, has also joined as Senior VP-Global Delivery. Setia was part of the executive leadership team at GE. His main areas of expertise are in incubating new businesses, P&L leadership, business transformation, revenue marketing and finance management. Tewari, in his most recent experience as COO of Quikr, was part of the team that has built India’s largest horizontal classifieds with deep vertical focus into real estate, jobs, goods, services and automobiles. Prior to Quikr, he was managing director at BlackRock in Gurugram.
Sridhar Desikan of Dr. Reddy’s joins Piramal Pharma Solutions in a global role
Dr. Reddy’s Head of Technology Strategy & Innovation, Dr. Sridhar Desikan, has joined Piramal Pharma Solutions as Global Technical Head of Formulations R&D. He will be based in the United States and will lead R&D teams across the globe. Before DRL, he worked with Biocon where he was part of the team to establish Biocon’s BMS R&D center. He was also with Dupont Pharma and Novartis as a Research Investigator earlier in his career. He completed his Chemical Engineering from BITS Pilani along with Masters and PhD from the United States.
TALENT INSIGHTS
Indian Pharma industry has always primarily focused on generics as a business model. Some companies have tried to go the innovation route but it has yielded limited results. The pipeline of new molecules has come down, thereby reducing opportunities in generics.
COVID-19 has led to an increased demand for Digital Transformation and Manufacturing professionals. In addition, with new product launches being postponed, Life Cycle Management (LCM) is a major area where increasing number of companies are focusing.
Top areas where we are seeing an increased talent demand are the following:
Manufacturing
Scaling up is one of the major areas where talent is needed and a lot of companies are gearing up for the challenge.
Technology and Digital Transformation
COVID-19 has spurred a sharp rise in demand for specialists with expertise in new-age technologies along with cross functional experience in Pharma.
Analytics
Analytics is here to stay and more and more industries are implementing it in their plans. This holds true for both Product Development and Marketing/Sales Force Effectiveness.
Regulatory and Govt Affairs
With the pricing pressure likely to continue for next few quarters, this is a strong area that has emerged and many companies are strengthening their expertise.
Business Development/Licensing
With the generics market not giving the right growth, companies are moving towards collaborations and licensing opportunities. Deal makers in this area are in high demand.
Medical Affairs and Real World Evidence
This is another area that is seeing an uptrend given the importance of Real World Evidence and HEOR.
Marketing
With the digital boom, Marketing is seeing an overhaul and is seeing a dynamic shift. Companies are increasingly focusing on newer ways to maximize their marketing and outreach.
References
- EMA Partners – Research Team
- Fierce Pharma News
- World Pharma News
- European Pharmaceutical Review
- Express Pharma News
- ET Markets & ET Prime
- Moneycontrol
- Company Annual Reports
Disclaimer: The information presented has been obtained through multiple sources including those provided in the bibliography section and is subject to change/validation. While every effort has been made to ensure the accuracy of the information provided, EMA Partners does not explicitly guarantee the accuracy of the information. EMA Partners does not accept any liability for any decisions made on the basis of the information provided.
Insights
Our Insights are the research and leadership trends that will benefit both clients and candidates, and inspire them to become better professionals

